All Products
Fusion Flux
Product Selection
When choosing a Lithium Borate Flux for your XRF or ICP application there are a number of factors to be considered:
Melting Point
The melting point of the flux should be sufficiently high so that the sample is fully dissolved but not too high that it causes volatilisation. This is particularly important for volatile compounds such as those containing Sodium (Na), Potassium (K) and Sulfur (S).
Purity
The purity of the flux is important in minimising sample contamination and background intensities. Where trace level accuracy is required (particularly for ICP), the purity of the flux is critical.
Hygroscopy
The level of moisture retention in the flux can have a significant impact on the weighing accuracy and fusion process. In general, pre-fused fluxes minimise water absorption over extended periods.
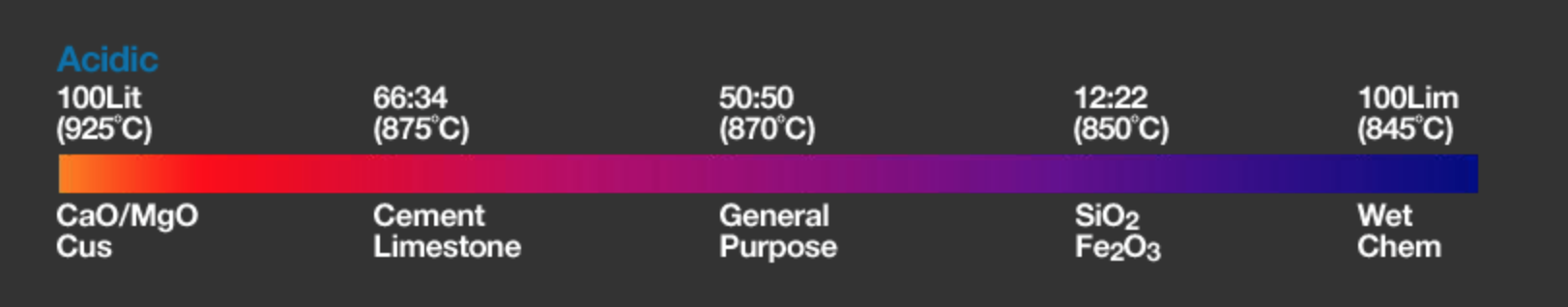
Acidity/Basicity
The choice of flux depends on the acidity/basicity of the sample and ideally the combination of flux and sample should be as close to neutral as possible.
Surface Area
Different flux manufacturing processes, although chemically identical, have different physical structures. Beaded, granular and powder are some of the more common types. The flux type has a large impact on the hygroscopy, weighing accuracy and melting rates. A pre-fused flux with a controlled particle size and low dust will provide the optimum conditions for fusion.
Additives
There are a number of additives that can be added to the flux to improve the overall outcome:
- Oxidant: this helps ensure that full oxidation of all compounds takes place either before flux melting or during the fusion process. It is important in reducing chemical attack of platinum labware and attaining a successful fusion bead.
- Releasing agent: certain samples can stick to the mould, a releasing agent (non-wetting agent) assists with the process by increasing the surface tension of the molten mix, thereby assisting with release.
XRF Scientific provides flux products that can meet your requirements regardless of which of these factors is most important to you. The chart below provides a recommendation of which flux to use for a range of common samples. For more specialist applications we are always happy to provide support and can often devise a custom solution which is just right for you.