Genetic testing is a helpful tool to diagnose genetic conditions by identifying the changes, commonly known as mutations or variants, in the patient’s DNA. Since our DNA is passed down from one generation to the next, it is important for an individual to understand how their condition will affect their way of life and future offspring. Pre- and post-genetic counseling from a trained genetic counselor are required steps when availing a genetic test as they help patients in answering questions pertaining to their conditions.
Genetic Testing Process in the Philippines
In the Philippines, genetic testing is usually performed after consultation with a qualified genetic counselor. A genetic counselor will review personal and family history, conduct disease risk assessment, and explain the benefits and risks associated with genetic testing. Once the patient fully understands and agrees to proceed with the test, the genetic counselor will endorse them to a doctor to provide a test prescription. Sample collection will be done at an affiliated local laboratory*. Most genetic tests are currently outsourced to different laboratories abroad. Centogene’s laboratory in Rostock, Germany is one of the service providers that specializes in genetic testing for rare genetic diseases. Once the result is released, post-genetic counseling is performed to discuss and review the medical implications of the genetic test results.
*most patients are endorsed at the Molecular Research and Diagnostic Unit-Service of the Institute of Human Genetics, UP Manila National Institutes of Health

Do I need Genetic Testing?
Genetic testing should be considered when:
- You have a family history of a genetic condition
- You might have an increased risk of developing a genetic condition
- You develop signs or symptoms of a genetic condition
- You might pass on the genetic condition to your children
- You are pregnant and have reason to believe that your baby is at risk of having a disease-causing mutation
What are the benefits and limitations of genetic testing?
BENEFITS
Genetic testing can confirm the presence of disease-causing mutations in an individual or alleviate unnecessary concerns. It allows people to make informed decisions about lifestyle, reproductive health, and the management and treatment of their condition.
LIMITATIONS
Research on variants of unclear significance is limited, meaning their effects are not yet well understood. This uncertainty can cause anxiety about the future or feelings of guilt and frustration. However, as research in genetics, medicine, and human health continues to advance, the clinical significance of these variants may be confirmed or disproven over time.
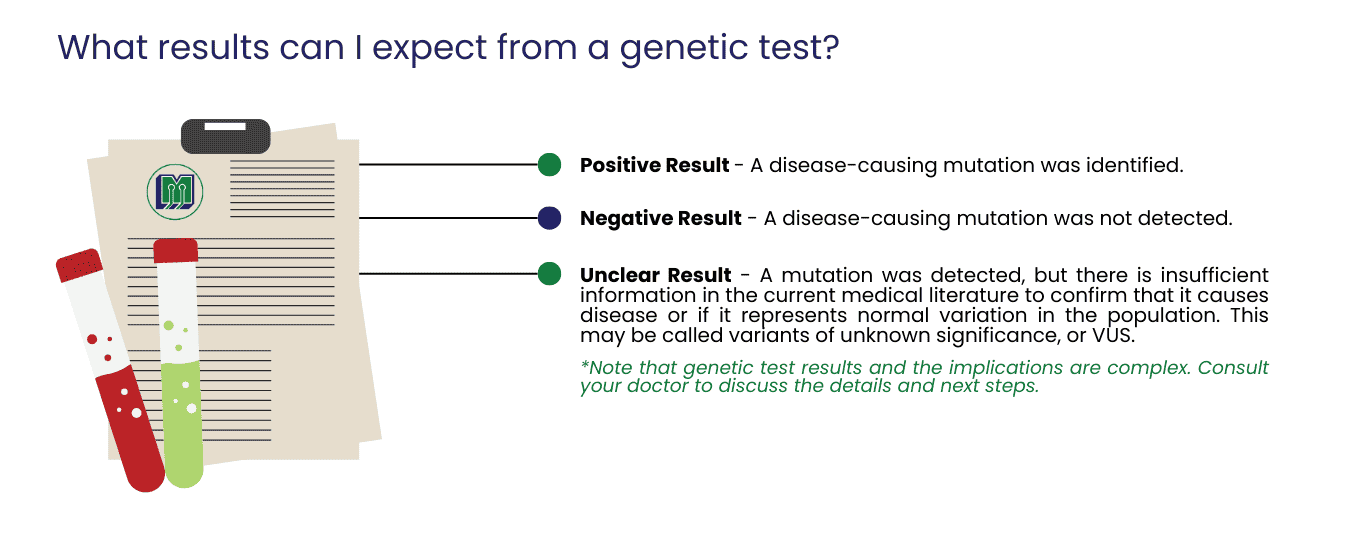
Are there risks associated with genetic testing?
In general, genetic testing poses minimal physical risk. Most tests are conducted using whole blood, except in certain prenatal and oncology-related cases. However, genetic testing can also involve emotional, social, and financial risks. It is advisable to discuss all potential risks, benefits, and limitations with your doctor before proceeding with a genetic test.
WHAT TYPE OF GENETIC TEST DO I NEED?
There are several types of genetic tests. Some involve looking for mutations in specific genes, others involve searching the whole genome. It is important to discuss with your physician which is the most appropriate test for you.
References:
Centogene. (2022). Genetic Testing. https://www.centogene.com/patient/genetic-testing
Centogene. (2022). Cheatsheet for Our Patients: Questions to Ask Your Physician. https://www.centogene.com/fileadmin/patients/downloads/centogene_patients_downloads_cheatsheet_v2.1eng_web_20220509.pdf
Medicover Genetics Editorial Team. (2022). What is a genetic test – benefits, limitations & how to choose the right type. https://medicover-genetics.com/what-is-a-genetic-test-benefits-limitations-how-to-choose-the-right-type/
Mayo Clinic Staff. (2020). Genetic testing. https://www.mayoclinic.org/tests-procedures/genetic-testing/about/pac-20384827
Number of individuals tested in the Philippines
Active physicians in our network
Genes covered in testing portfolio
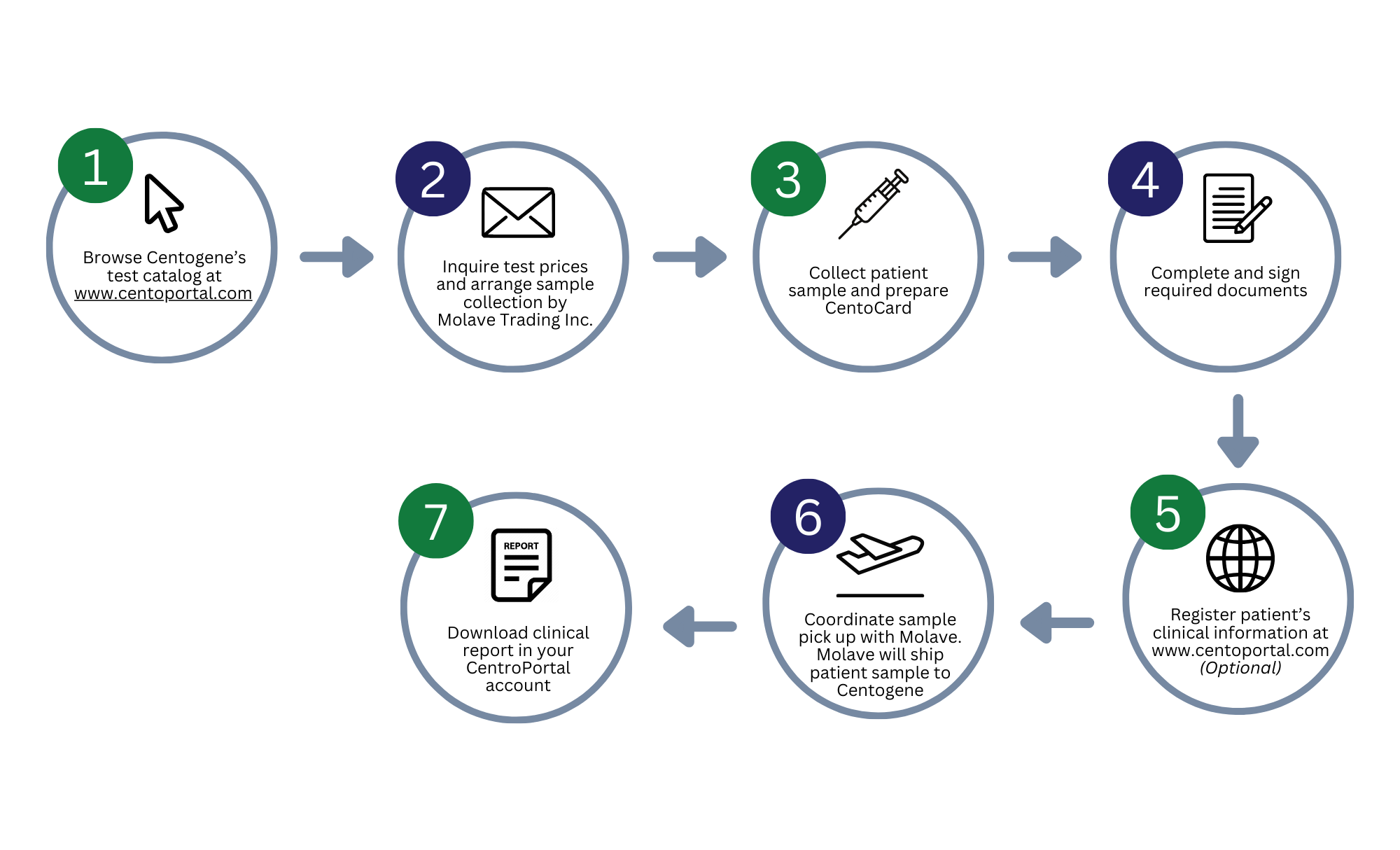
CENTOGENE ORDER WORKFLOW
FREQUENTLY ASKED QUESTIONS
How can I avail of your genetic testing services?
We only accept test requests ordered by a doctor. Please consult with your doctor for testing recommendations and pre-genetic counseling.
What is the process for sample collection?
Sample collection is usually arranged and conducted at the requesting physician’s clinic or referred to the Service Arm of Molecular Research and Diagnostic Unit of Institute of Human Genetics at the University of the Philippines Manila – National Institutes of Health. We only facilitate sample pick-up and shipment to Centogene.
What are the sample requirements?
| Test |
Accepted Material |
| Whole Genome Sequencing
Whole Exome Sequencing Next-Generation Sequencing Panels Chromosomal Microarray Analysis Single Gene Analysis |
1 full CentoCard filter card (10 complete blood spots), stored and shipped at ambient temperature, kept dry and away from sunlight. – preferred material |
| ≥1 ml EDTA blood, stored and shipped at ambient temperature | |
| Buccal swab (Oragene ORAcollect DX OCR-100 or OCD-100 collection kit) | |
| Saliva (Oragene OG-600/610/675 or OG-500/510/575 collection kit) | |
| Extracted DNA (≥ 1 ug, recommended extraction through Qiagen kits, eluted in water or AE buffer in 20 ul minimum volume, plus evaluation of 260/280 ratio (1.6-2.1) and fluorescent dye quantification of concentration (> 10 ng/ul) before sending. | |
| Multiomic Testing (MOx)
CentoMetabolic MOx, CentoXome Mox, and CentoGenome MOx |
2 full CentoCard filtercards (each with 10 complete blood spots), stored and shipped at ambient temperature, kept dry and away from sunlight. – preferred material |
| 1 full CentoCard filtercard (10 complete blood spots) + 2 ml EDTA blood | |
| Prenatal Testing | Amniotic fluid (minimum of 10 ml placed in a sterile conical tube and safely sealed. Amniotic fluid must be clear and without visible blood contamination. |
| Chorionic villi (minimum of 10 cleaned villi in a minimum of 35 ml of RPMI transport medium with fetal calf serum, container must be safely sealed. Chorionic villi must be clean and white; no maternal material should be present (i.e. no placenta)). | |
| Cord blood (≥1 ml) | |
| Extracted DNA (≥ 1 ug, recommended extraction through Qiagen kits, eluted in water or AE buffer in 20 ul minimum volume, plus evaluation of 260/280 ratio (1.6-2.1) and fluorescent dye quantification of concentration (> 10 ng/ul) before sending. | |
| Additional Material for products of Conception/Pregnancy Loss (POC) | Biopsy material (≥ 20 mg submerged in RPMI transport medium with fetal calf serum, container must be safely sealed). |
| Tissue sample (≥ 20 mg submerged in RPMI transport medium with fetal calf serum, container must be self-sealed). | |
| Maternal Contamination Marker (mother’s sample) | 1 full CentoCard filter card (10 complete blood spots), stored and shipped at ambient temperature, kept dry and away from sunlight. – preferred material |
What is included in the genetic testing fee?
Test fee includes the genetic analysis, sample shipment, and processing fees (i.e. Bureau of Quarantine Certificate).
What are your payment options?
Payment may be settled through bank deposit or online bank transfer through any of our company’s bank accounts. Payment through credit card is currently unavailable.
How long will it take to receive the test results?
The turnaround time varies depending on the type of test and includes shipping time from the Philippines to Germany, which typically takes around 5-7 days.
|
Test |
Estimated Turnaround time* |
| Whole Genome Sequencing |
5-6 weeks |
| Whole Exome Sequencing |
7-8 weeks |
| NGS Panels |
6-7 weeks |
| Chromosomal Microarray |
4-5 weeks |
| Single Gene Testing (Sanger Sequencing, qPCR, MLPA, repeat expansion analysis, targeted carrier testing) |
4-5 weeks |
| Enzyme/Biomarker analysis |
3 weeks |
*Please note that these are only estimates, as additional confirmatory analysis may be performed in some cases, thereby lengthening the turnaround time.
How can I get my results?
Test results are directly sent to the requesting physician. Please contact your doctor for post-genetic counseling.
I am located outside of Metro Manila. How can I avail of the service?
We can send CentoCards to your location; however, please note that we do not facilitate sample collection. We can provide instructions to your requesting physician on how to collect and prepare the sample. CentoCards must be kept dry and away from sunlight, shipped at room temperature and sent to our office address (see contact information below). Once we receive the sample, we will then facilitate the shipment to Centogene.
Ano ang Genetic Testing?
Ang ‘genetic testing’ ay isang pamamaraang nagsusuri sa mga kondisyong may kaugnayan sa ‘genes’ ng isang indibidwal. Ito ay may kakayahang tumukoy ng mga pagbabago, karaniwang tinatawag na ‘mutations’ o ‘variants’ sa Inglés, sa loob ng DNA ng isang pasyente. Ang DNA, o ‘deoxyribonucleic acid’ naman sa Inglés, ay naglalaman ng mga sangkap na siyang ginagamit sa pagbuo at pag-unlad ng buhay. Dahil sa ang DNA ay naipapasa mula sa magulang patungo sa anak, mahalaga para sa isang indibidwal na maunawaan kung paano nito maaaring maapektuhan ang kanilang kondisyon, uri ng pamumuhay, at mga magiging anak. Nararapat lamang na magpakonsulta sa isang ‘genetic counselor’ bago magsagawa ng genetic test, sapagkat sila ang may angkop na kakayahang magpaliwanag at sumagot sa mga katanungang nauukol sa kondisyong medikal na namamana.
Mga Hakbang Ukol sa Genetic Testing sa Pilipinas
Ang genetic testing sa ating bansa ay karaniwang ginagawa pagkatapos sumailalim sa paunang pagsusuri, o tinatawag nating ‘pre-genetic counseling’ sa medikal na termino, ng isang genetic counselor. Ang genetic counselor ang siyang pangunahing nagsusuri ng kasaysayang medikal ng pasyente at ng kanyang pamilya, at nagpapaliwanag ng mga benepisyo at maaaring maging panganib dulot ng genetic testing. Kapag ganap nang naintindihan ang konsepto at sumang ayon na magpatuloy sa genetic test, bibigyan ang pasyente ng resetang naaangkop sa pagsusuri. Ang pagkuha ng sample ay karaniwang ginagawa sa isang lokal na laboratoryo*; samantalang ang mga genetic test ay kasalukuyan namang ipinapadala sa iba’t ibang laboratory sa ibang bansa. Ang Centogene Laboratory ay isa sa mga laboratoryo na gumagawa ng genetic testing para sa mga sakit na may kinalaman sa DNA at ito ay matatagpuan sa Rostock, Germany. Kapag nailabas na ang resulta, isinasagawa ang ‘post-genetic counseling’ upang talakayin at suriin ang mga implikasyong medikal na maaaring maging bunga ng genetic testing.
*Karamihan ng mga pasyente ay ipinapadala sa Molecular Research and Diagnostic Unit-Service ng Institute of Human Genetics, UP Manila National Institutes of Health.

Kailangan ko ba magpa-genetic test?
Ang genetic testing ay isinasagawa lamang ayon sa mga sumusunod na pamantayan:
- Ang pasyente ay mayroong kasaysayang medikal sa pamilya na may kaugnayan sa genes o namamanang sakit
- Ang pasyente ay mayroon mas mataas na probabilidad upang magkaroon ng isang kondisyong namamana
- Ang pasyente ay nagkaroon ng mga sintomas ng isang namamanang sakit
- Ang kondisyon ay maaaring maipasa sa iyong mga anak
- Ang pasyente ay buntis at naniniwalang ang pinagbubuntis na sanggol ay nasa panganib ng kondisyong medikal
Ano ang mga benepisyo at limitasyon ng genetic testing
BENEPISYO
Isa sa mga pangunahing benepisyo ng genetic testing ay ang pagkakaroon ng kasagutan kung ang nararanasang sakit ay bunga ng mutation sa DNA. Ang resulta ng pagsusuri ay maaaring magpagaan ng agam-agam na karaniwang nararanasan ng mga pasyenteng may kondisyong medikal. Ito rin ay nagbibigay-daan upang makagawa ng angkop na pagpapasya hinggil sa uri ng pamumuhay, kalusugang reproduktibo, at wastong gamutan ng kanilang kondisyon.
LIMITASYON
Marami sa mga variant na karaniwang makikita sa resulta ng genetic testing ay hindi pa rin lubos na nauunawaan ang epektong medikal. Ang kawalang-katiyakang ito, sa kasamaang palad, ay may posibilidad na magbunga ng patuloy na pagkabahala sa mga pasyente at magulang. Gayunpaman, habang patuloy na umuunlad ang pananaliksik sa larangan ng medisina, ang mga variant na ito ay maaaring magkaroon ng naaangkop na kasagutan sa hinaharap.

Mayroon bang mga panganib na kaakibat ang genetic testing?
Karaniwan sa mga genetic test na nangangailangan ng dugo, maliban sa mga kasong may kinalaman sa prenatal at kanser, ay may maliit lamang na porsyento ng panganib. Gayunman, ang genetic testing ay may kaakibat na suliranin tulad ng emosyonal, sosyal, at pinansyal na isipin. Mas mainam na talakayin ang mga potensyal na panganib, benepisyo, at limitasyon sa iyong doktor bago magpatuloy sa genetic test.
ANONG URI NG GENETIC TEST ANG KAILANGAN KO?
Mayroong iba’t ibang uri ng genetic tests ang maaaring magawa sa pasyente. Ang ilan sa mga ito ay ginagamit sa paghahanap ng mga partikular na mutation sa mga gene; samantalang ang iba naman ay naghahanap ng mga pagbabago sa kabuuang genome ng isang indibidwal. Mainam na talakayin sa iyong geneticist kung alin ang pinakaangkop na pagsusuri para sa iyo.
References:
Centogene. (2022). Genetic Testing. https://www.centogene.com/patient/genetic-testing
Centogene. (2022). Cheatsheet for Our Patients: Questions to Ask Your Physician. https://www.centogene.com/fileadmin/patients/downloads/centogene_patients_downloads_cheatsheet_v2.1eng_web_20220509.pdf
Medicover Genetics Editorial Team. (2022). What is a genetic test – benefits, limitations & how to choose the right type. https://medicover-genetics.com/what-is-a-genetic-test-benefits-limitations-how-to-choose-the-right-type/
Mayo Clinic Staff. (2020). Genetic testing. https://www.mayoclinic.org/tests-procedures/genetic-testing/about/pac-20384827
Number of individuals tested in the Philippines
Active physicians in our network
Genes covered in testing portfolio
CENTOGENE ORDER WORKFLOW
MGA MADALAS ITANONG
Paano ako makakapag-avail ng inyong genetic testing?
Tumatanggap lamang kami ng mga genetic test na nireseta ng isang geneticist. Mangyaring kumunsulta sa iyong geneticist para sa mga rekomendasyon sa pagsusuri at pre-genetic counseling.
Ano ang mga kinakailangan sa sample?
Mayroong iba’t ibang uri ng genetic tests ang maaaring magawa sa pasyente. Ang ilan sa mgito ay ginagamit sa paghahanap ng mga partikular na mutation sa mga gene; samantalang ang iba naman ay naghahanap ng mga pagbabago sa kabuuang genome ng isang indibidwal. Mainam na talakayin sa iyong geneticist kung alin ang pinakaangkop na pagsusuri para sa iyo.
Ano ang mga kinakailangan sa sample?
| Test |
Accepted Material |
| Whole Genome Sequencing
Whole Exome Sequencing Next-Generation Sequencing Panels Chromosomal Microarray Analysis Single Gene Analysis |
1 full CentoCard filter card (10 complete blood spots), stored and shipped at ambient temperature, kept dry and away from sunlight. – preferred material |
| ≥1 ml EDTA blood, stored and shipped at ambient temperature | |
| Buccal swab (Oragene ORAcollect DX OCR-100 or OCD-100 collection kit) | |
| Saliva (Oragene OG-600/610/675 or OG-500/510/575 collection kit) | |
| Extracted DNA (≥ 1 ug, recommended extraction through Qiagen kits, eluted in water or AE buffer in 20 ul minimum volume, plus evaluation of 260/280 ratio (1.6-2.1) and fluorescent dye quantification of concentration (> 10 ng/ul) before sending. | |
| Multiomic Testing (MOx)
CentoMetabolic MOx, CentoXome Mox, and CentoGenome MOx |
2 full CentoCard filtercards (each with 10 complete blood spots), stored and shipped at ambient temperature, kept dry and away from sunlight. – preferred material |
| 1 full CentoCard filtercard (10 complete blood spots) + 2 ml EDTA blood | |
| Prenatal Testing | Amniotic fluid (minimum of 10 ml placed in a sterile conical tube and safely sealed. Amniotic fluid must be clear and without visible blood contamination. |
| Chorionic villi (minimum of 10 cleaned villi in a minimum of 35 ml of RPMI transport medium with fetal calf serum, container must be safely sealed. Chorionic villi must be clean and white; no maternal material should be present (i.e. no placenta)). | |
| Cord blood (≥1 ml) | |
| Extracted DNA (≥ 1 ug, recommended extraction through Qiagen kits, eluted in water or AE buffer in 20 ul minimum volume, plus evaluation of 260/280 ratio (1.6-2.1) and fluorescent dye quantification of concentration (> 10 ng/ul) before sending. | |
| Additional Material for products of Conception/Pregnancy Loss (POC) | Biopsy material (≥ 20 mg submerged in RPMI transport medium with fetal calf serum, container must be safely sealed). |
| Tissue sample (≥ 20 mg submerged in RPMI transport medium with fetal calf serum, container must be self-sealed). | |
| Maternal Contamination Marker (mother’s sample) | 1 full CentoCard filter card (10 complete blood spots), stored and shipped at ambient temperature, kept dry and away from sunlight. – preferred material |
Ano ang kasama sa bayad para sa genetic testing?
Kasama sa bayad ng genetic test ang genetic analysis, bayad sa pagpapadala ng sample, at mga processing fees (hal. Pagproseso ng Bureau of Quarantine Certificate).
Ano ang inyong mga opsyon sa pagbabayad?
Ang pagbabayad ay maaaring gawin sa pamamagitan ng bank deposit o online bank transfer sa alinman sa mga bank account ng aming kumpanya. Sa kasalukuyan, hindi pa available ang pagbabayad gamit ang credit card.
Gaano katagal bago makuha ang resulta ng test?
Ang paglabas ng resulta ay nag-iiba ayon sa uri ng test na gagawin. Kasama na rito ang pagpapadala ng sample mula sa Pilipinas patungong Germany, na karaniwang tumatagal ng humigit-kumulang 5-7 na araw.
|
Test |
Estimated Turnaround time* |
| Whole Genome Sequencing |
5-6 weeks |
| Whole Exome Sequencing |
7-8 weeks |
| NGS Panels |
6-7 weeks |
| Chromosomal Microarray |
4-5 weeks |
| Single Gene Testing (Sanger Sequencing, qPCR, MLPA, repeat expansion analysis, targeted carrier testing) |
4-5 weeks |
| Enzyme/Biomarker analysis |
3 weeks |
*Mahalagang paalala: Ang mga ito ay pagtatantiya lamang. Maaaring magsagawa ng karagdagang confirmatory analysis sa ilang mga kaso na siyang magpahaba ng turnaround time.
Paano ko makukuha ang aking mga resulta?
Ang mga resulta ng pagsusuri ay direktang ipinadala sa doktor na nagpagawa ng pagsusuri. Mangyaring makipag-ugnayan sa iyong doktor para sa post-genetic counseling.
Ako ay nakatira sa labas ng Metro Manila. Paano ako makakapag-avail ng inyong serbisyo?
Maaaring ipadala ang CentoCards sa inyong kinaroroonan; gayunpaman, hindi kami nagsasagawa ng pagkuha ng sample. Maaari naming ituro ang mga wastong hakbang sa pagkuha at paghahanda ng sample sa iyong doktor. Ang CentoCards ay pinapanatiling tuyo at malayo sa sikat ng araw; ipadala ito sa aming opisina gamit ang contact information na makikita sa ibaba. Kapag natanggap na namin ang sample, kami na ang mag-aasikaso ng pagpapadala nito sa Centogene laboratory.
TESTIMONIALS

GET IN TOUCH

Bea Angela Acosta
bea_acosta@acerogroup.org

Rae Anne Añonuevo
rae_anonuevo@acerogroup.org

Jessica Biwang
jessica_biwang@acerogroup.org
MESSAGE US

Molave Trading Inc.
891 EDSA Brgy. South Triangle Diliman, Quezon City 1103
(+632) 8660-5253 to 54, 8924-2225, (+63) 917-8565858, (+63) 923-737021
DATA PROTECTION POLICY
We care about the confidentiality of your personal data. Centogene and Molave Trading Inc. respect your data protection rights and comply with all applicable laws and regulations regarding data protection and data privacy. Both companies are governed by the European General Data Protection Regulation (GDPR) and the Philippine Republic Act 10173 also known as the Data Privacy Act of 2012, respectively.